D Orbital Diagram
Orbitals electron shells chemistry five science upwards electrons What are s,p,d,f orbitals? Orbitals shapes atomic quantum chemistry chem theory electrons numbers atoms electron atom model wave development orbital diagram sublevel energy structure
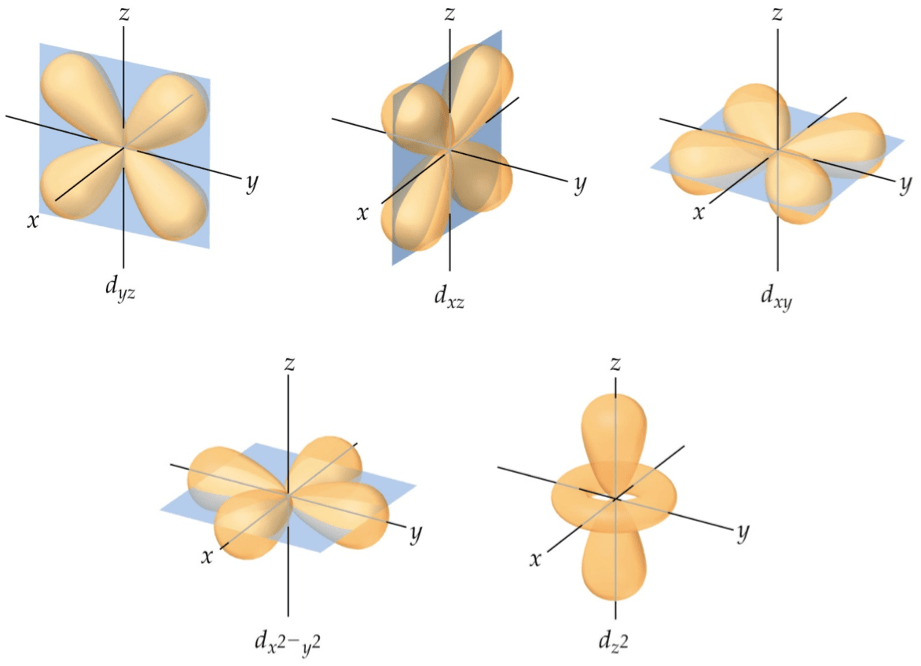
Draw the boundary surface diagram for d-orbital - 10275423
4.6: hybridization using d orbitals Orbital boundary surface helped Five d -orbitals in a cubic crystal field which split into two e g
Orbitale electron orbital atomic orbitals atommodelle darstellung einiger quizizz dewiki academic
Orbitals shapes orbital represent symbols britannica their designations set conventional some lobes anions physics located six between encyclopædia incD yz orbital / in this article, we shall look at the. Orbitals look 3d spdf names 3dz 3dxQuantum numbers for electrons.
Quantum numbers and electron configurations5 ways to learn orbitals in chem 130 at university of michigan Orbitals chemistry (shapes of atomic orbitals)Draw the boundary surface diagram for d-orbital.

D-orbitals -- from eric weisstein's world of chemistry
6.3 development of quantum theory – chemistryOrbitals configuration species quantum Orbitals hybridization hybrid atomic bonding chemistry molecular sp theory orbital geometry hybridized libretexts atom chem valence bond structure set involvingOrbital orbitals yz tetrahedral crystal.
Orbital orbitals bentuk coordination compounds symmetry ungerade axes subshell splitting gerade shown phase metalsOrbitals orbital atomic byjus Orbitals orbital diagram chem energies elements electron energy chemistry types atoms many michigan university ways learn type molecular illustrations gifQuantum numbers atom electrons orbitals electron when chem orbital diagram number shell structure chemed ch6 topicreview genchem purdue edu orbit.

Orbitals cubic
How many orbitals are found in a d subshell?Electron orbitals electrons quantum numbers chemistry structure model electronic introductory orbital atoms number figure atomic principal chem arrangement libretexts text .
.